How To Determine If A Chemical Equation Is A Redox Reaction
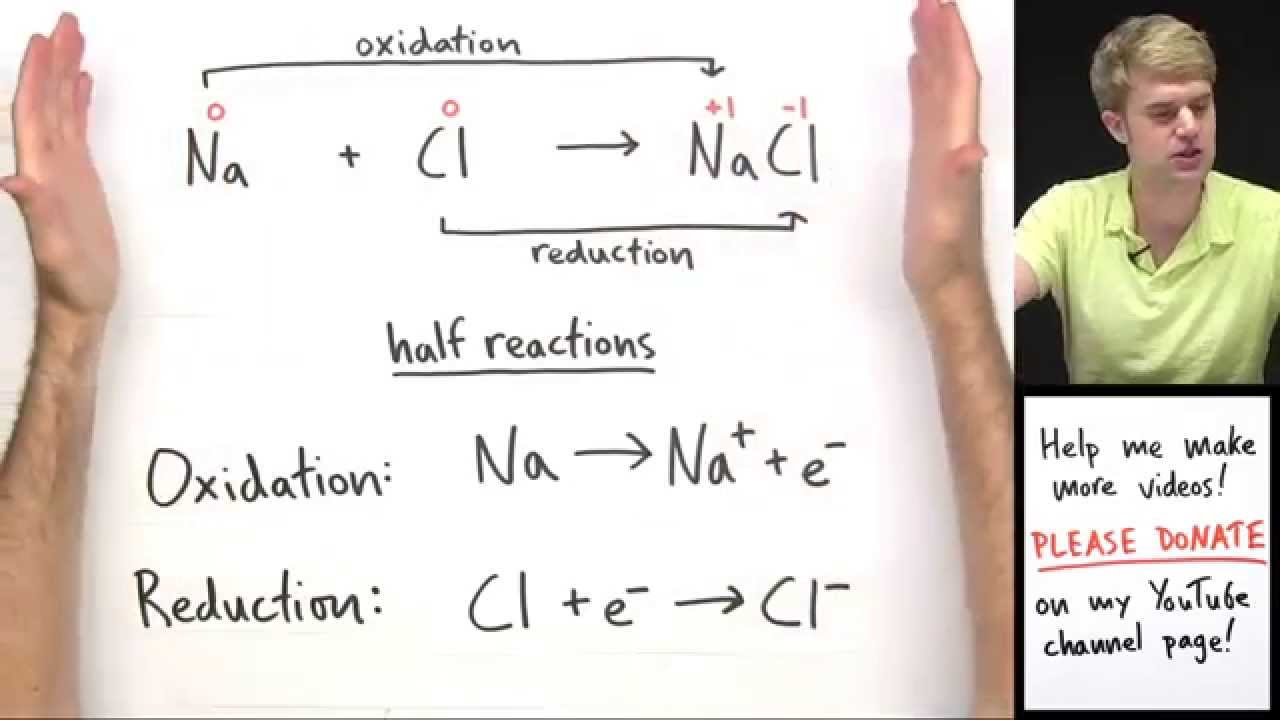
Regents redox reactions have a Free element on one side of a reaction and the same element bonded to something on the other side. A g N O X 3 N a C l A g C l N a N O X 3 B.

Oxidation Reduction Reaction Redox Reaction Ppt Video Online Download
The ON of a monatomic ion is the same as its charge examples are Na 1.
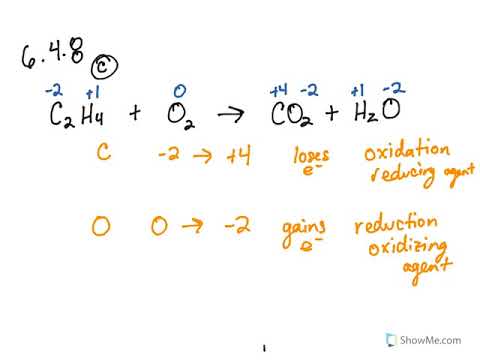
How to determine if a chemical equation is a redox reaction. The reaction is a redox process. B a C l X 2 K X 2 C O X 3 B a C O X 3 2 K C l C. The charges dont match yet so this is not a balanced equation.
Chemical equations of redox reactions can be balanced by using any one of the following methods. The MnO4 on the left has a -1 charge and the 8 hydrogens add a 8 charge adding up to a 7 charge on the left overall. This is a chemical equation that must be balanced for charge as well as mass.
Discusses methods for identifying redox reactions from chemical equations. Redox reactions are oxidation-reduction chemical reactions in which the reactants undergo a change in their oxidation states. The first step in solving any redox reaction is to balance the redox equation.
C u O C O C u C O X 2 D. SO 2 has been oxidized by MnO 4 and so MnO 4 is the oxidizing agent. Identifying Redox Reactions For the Regents exam they only ask you to find a reaction that an element changes oxidation number.
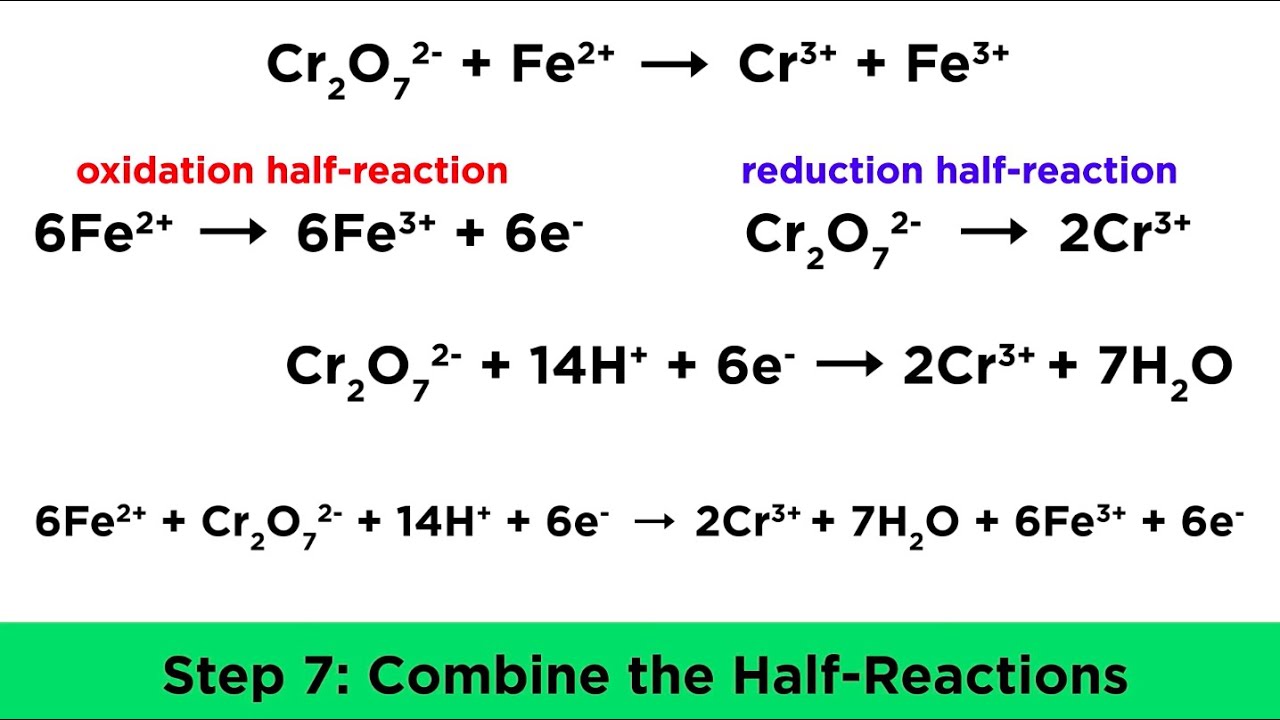
We can use each half-reaction to balance the charges. Recombine the half-reactions to form the complete redox reaction. The sum of all ONs in.
Write the skeletal equation of all the reactants and products of the reaction. The term redox is a short form of reduction-oxidation. To identify a redox reaction we must first calculate the oxidation number of each atom in the reaction.
A The appropriate oxidation numbers are The only atoms which change are Mn from 7 to 2 a reduction and S from 4 to 6 an oxidation. Balancing of Chemical Equations of Redox Reactions. Notice that the Cl-ions drop out as they are spectator ions and do not participate in the actual redox reaction.
On the other hand if the reaction. If there is a change in oxidation number then the reaction is a redox reaction. One example is the burning of methane the principal component of natural gas Figure 56.
To balance out this charge they must be equal. A redox reaction is a chemical reaction in which reduction and oxidation occur. D shows an acid-base reaction.
The sum of all ONs in a neutral compound is zero. H C l K O H K C l H X 2 O. The answer key shows the correct answer as D but Im confused as to why.
If you look at the charges in step 4 the left side adds up to 7 and the right side adds up 2. The ON of an element in its free state is zero examples are Al Zn H O N. I dont understand how to identify a redox reaction.
The Mn on the right has a 2 charge so that is 2. All the redox reactions can be broken down into two different processes a. Combustion Reactions A combustion reaction is a type of redox reaction that occurs when a substance combines with molecular oxygen to make oxygen-containing compounds of other elements in the reaction.
Determine the oxidation states of the species involved. Once the redox equation is balanced use the mole ratio to find the concentration or volume of any reactant or product provided the volume and concentration. Equalize the electron transfer between oxidation and reduction half-equations.
This will balance the reaction in an acidic solution where there is an excess of H ions. If there is no change in oxidation number then the reaction is not a redox reaction. Lists ways to determine whether or not a given reaction is oxidation-reduction.
Balance the following redox reaction. 563 C H 4 2 O 2 C O 2 2 H 2 O. If the redox reaction takes place in an acidic medium proton or will appear in the chemical equation for redox reaction.
Example- 2H 2 O 2 -- 2H 2 O Free element --- Bonded element. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. In basic solutions there is an excess of OH - ions.
The following steps are involved in this method-i.
Redox Reactions Solutions Examples Activities Experiment Videos

Predicting Spontaneous Direction Of A Redox Reaction Introduction To Chemistry

Oxidation Reduction Reactions Principles Of Structural Chemistry
Oxidation Reduction Reactions Redox

Question Video Identifying A Spectator Ion In A Simple Redox Reaction Nagwa
Balancing Redox Reactions In Acidic And Basic Conditions Youtube

Redox Oxidation Reduction Reaction Definition Examples

Redox Oxidation Reduction Reaction Definition Examples

Oxidation And Reduction Reactions Basic Introduction Youtube
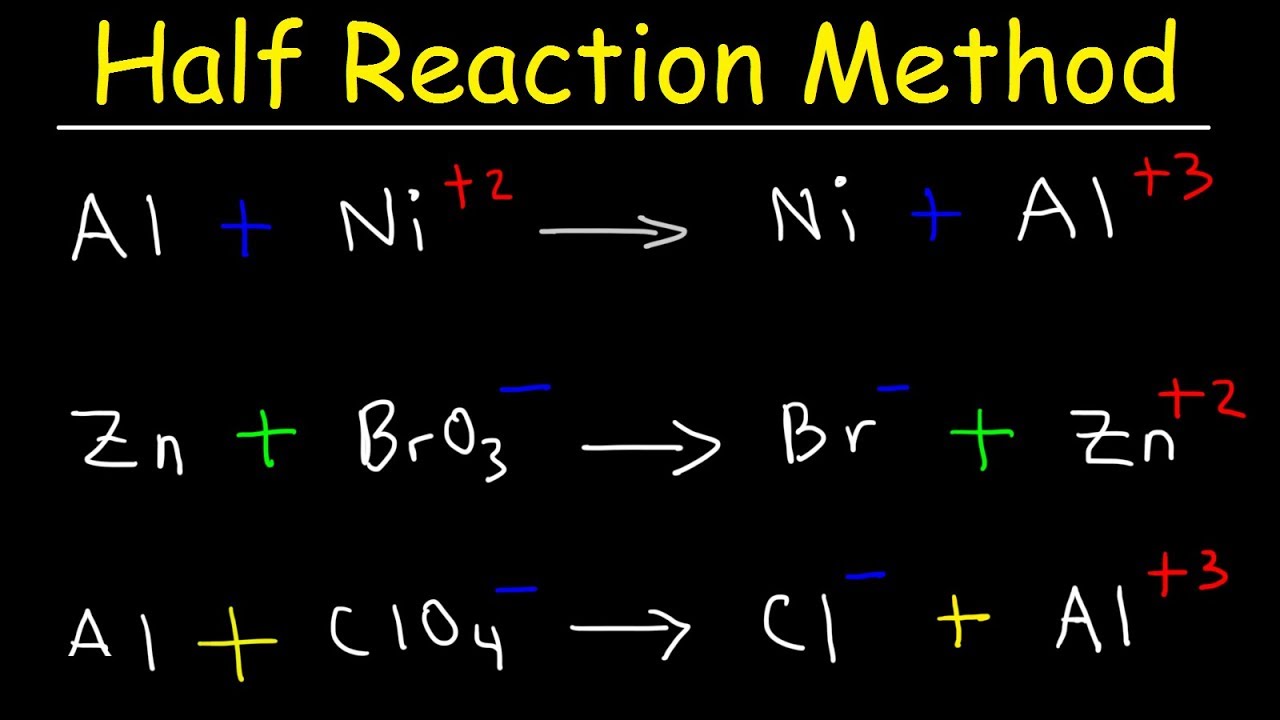
Half Reaction Method Balancing Redox Reactions In Basic Acidic Solution Chemistry Youtube

1 14 Cashing In On Redox Biology Libretexts

Oxidation Reduction Reaction Redox Reaction Ppt Video Online Download

Balancing A Redox Equation In Acidic Solution Worked Example Video Khan Academy
Oxidation And Reduction In Organic Chemistry Master Organic Chemistry

Spontaneity And Redox Reactions Video Khan Academy

6 5 Classifying Chemical Reactions Redox Problems Chemistry Libretexts
Post a Comment for "How To Determine If A Chemical Equation Is A Redox Reaction"