End Point Of Ebt Indicator
2 endpoint Eriochrome Black T indicator solution. Similar in its properties but much more stable solutions can be kept for up to a year is calmagite.
Https Www Canterbury Ac Nz Media Documents Science Outreach Magnesium Calcium Pdf
The ions involved in water hardness ie.
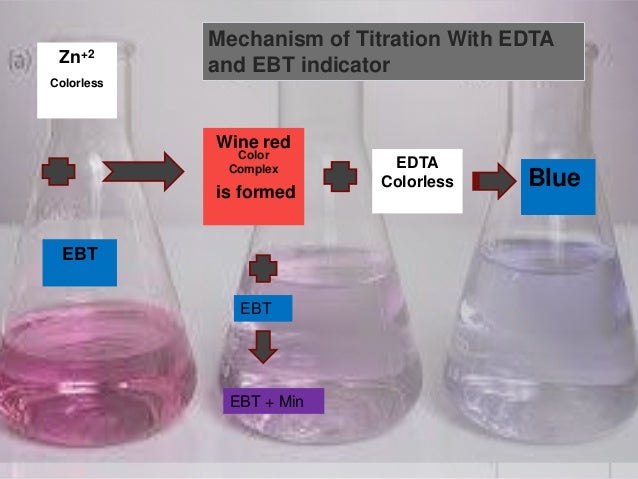
End point of ebt indicator. Those volumetric titrations or analysis in which the end point is indicated by a colored complex are known as complexometric titrations. When EDTA is titrated against the complex EDTA replaces all the EBT and forms a stable Ca Mg EDTA complex. An indicator capable of producing an unambiguous color change is usually used to detect the end-point of the titration.
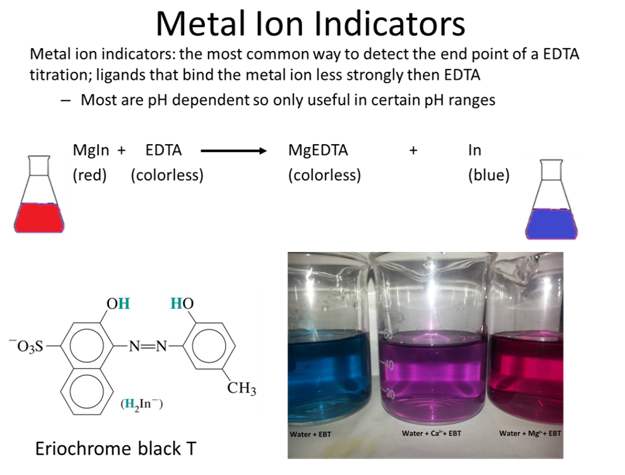
Eriochrome Black T exists as a wine-red complex when Mg2 is present in solution at pH 10. A drop of indicator is added in the start of the titration the endpoint has been appeared when color of the solution is changes. A substance that changes color of the solution in response to a chemical change.
The end point is detected by some physical change produced by the solution by itself or more usually by the addition of an auxiliary reagent known as an indicator. Phenolphthalein indicator used in acid-base titration. Such a titration experiment can be used to quantify the amount of cyanide present in a solution.
What are ion-exchange resins. It goes through first from the wine red to a purple which then becomes are deeper blue after. Having determined the average titre of the magnesium chloride solution determine the number of moles used.
In this type of titration an indicator is used which is capable of producing clear colour change in titration which indicates end point of the titration. Reference solutions will be available for color comparison. The end point of the reaction is indicated by the formation of a permanent precipitate or turbidity.
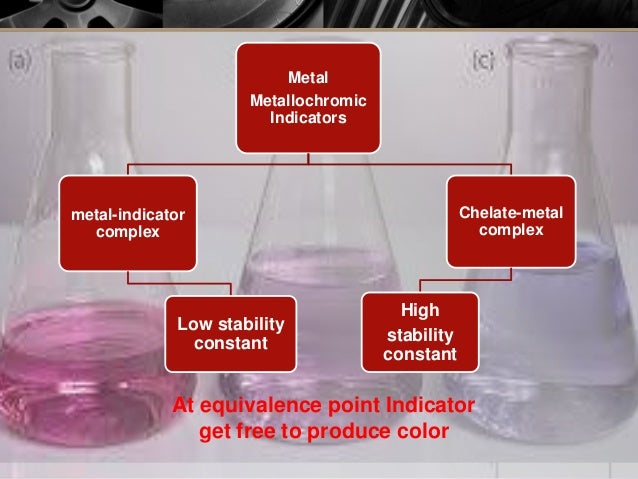
Ca Mg EBT EDTA Ca Mg EDTA EBT Wine redunstable Stable Steel blue 9. When the EDTA has chelated all the Mg2 present in solution the indicator free and uncomplexed to Mg2 will be robins egg blue. Eriochrome Black T titration - Concentration of Indicator and endpoint.
Be sure to read the buret to 001 mL. Given the Mg2. To carry out metal cation titrations using EDTA it is almost always necessary to use a complexometric indicator to determine when the end point has been reached.
Eriochrome Black T solutions are unstable so it is prepared as a solid mixed with NaCl 100 mg of indicator ground with 20 g of NaCl or as a fresh solution shelf life one day. Refill the buret read it and titrate the second solution. Common indicators are organic dyes such as Fast Sulphon Black Eriochrome Black T Eriochrome Red B Patton.
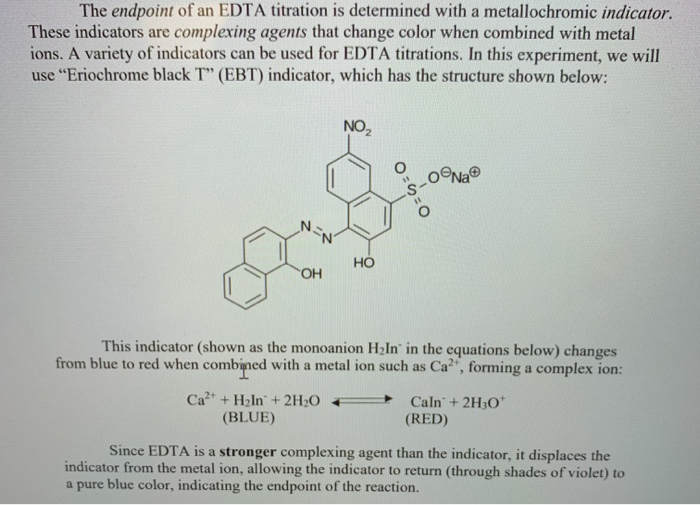
The endpoint of a complexometric EDTA titration using either Calmagite or EBT as the indicator is detected as the colour changes from pink to blue. The end point and the equivalence point may not be identical. There is EDTA s.
While not as frustrating an indicator as murexide Eriochrome Black Ts wine red to blue transformations provide significant challenges. Rule of thumb similar to that used for pH indicators tells that change of 120 mVn where n is number of electrons required to oxidize or reduce the indicator is in most cases enough for. Another term for a complexing agent.
The change from fully wine red to fully blue with no trace of red takes around 1mL. The liberated EBT indicates the end point as steel blue. It is also known as chelatometry.
Determination of Calcium in water samples using Eriochrome Black T as an indicator. It can be used instead of Eriochrome Black T in most titrations. It turns red when Ca2 ions are added.
This color change marks the endpoint. One of the most common methods for determination of endpoint owing to its simplicity least cost and accuracy. The endpoint detection in complexometric titration can be done by two methods.
Here cyanide is an example of a complexone. The initial color should be red and the endpoint color blue with no purple tint to it. EBT is blue in a buffered solution an aqueous solution consisting of a mixture of a weak acid and its conjugate base or vice versa at pH 10.
Complexometric titration are those reactions where a simple ion is transformed into a complex ion and the equivalence point is. End point is usually. EDTA ratio of 1.
Titrate the EDTA with the magnesium chloride solution until the endpoint is reached a permanent colour change from blue to pink. Near equivalence point change of redox potential of the solution is often in the range of 03-04 V. Add only very small quantities of the indicator are needed.
Involves using titration of calcium in water with EDTA. What are the indicators used in complexometric titration. Ca2 aq and Mg2 aq change the steel blue colour of EBT indicator into wine red colour.
Do a third titration if there is poor agreement between the first two.

Ebt Indicator Eriochrome Black T Pure Indicator Grade Acros Organics

Complexation Titration Chemistry Libretexts
Edta Chelation And Titrations Chemistry Libretexts

Complexometric Titrations With Edta
Solved 5 3 In Part 1 Of The Experiment Preparation Of Chegg Com
Complexometric Titrations With Edta
Eriochrome Black T Sciencemadness Wiki
Https Www Canterbury Ac Nz Media Documents Science Outreach Magnesium Calcium Pdf
Https Www Mtsu Edu Chemistry Chem2230 Pdfs Exp9 Pdf
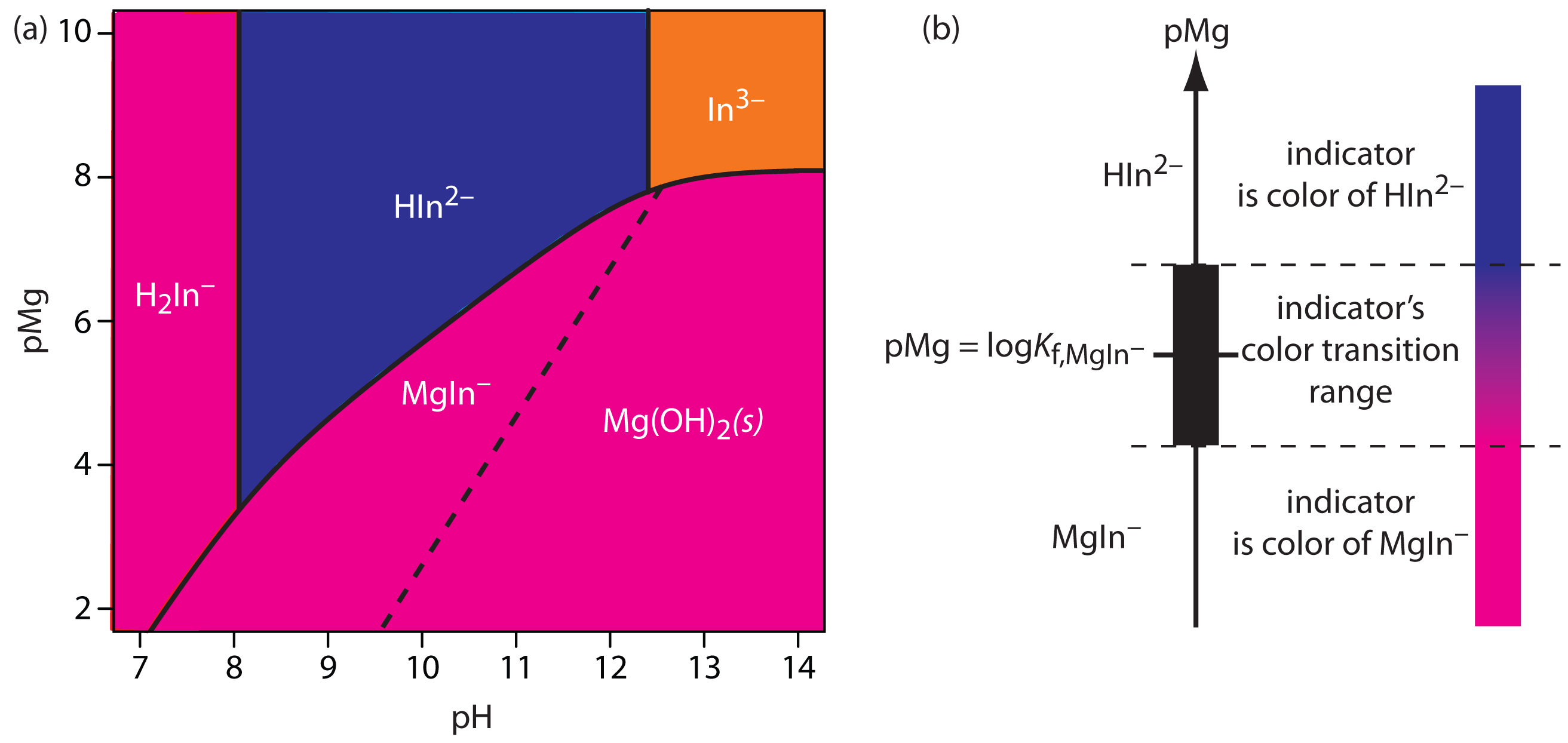
Complexation Titration Chemistry Libretexts
Http Faculty Sdmiramar Edu Fgarces Zcourse All Year Ch251 A Leclab 03 Inlab 07 Experiments 02 Titrationedta Metals Ba Titration Edta Ba Pdf

Solved Concepts The Most Common Indicator For Edta Titra Chegg Com

What Happens If Don T Use Mgcl In Buffer Solution In Ca Mg Titration Chemistry Stack Exchange

Complexometric Titrations With Edta

How To Determine Hardness Of Water By Edta Method Procedure And Problems On Hardness Youtube

Photometric Complexometric Titration Metrohm Blog
Http Faculty Sdmiramar Edu Fgarces Zcourse All Year Ch251 A Leclab 03 Inlab 07 Experiments 02 Titrationedta Metals Ba Titration Edta Ba Pdf

Post a Comment for "End Point Of Ebt Indicator"